科学文献
Aging is a key factor in the occurrence and progression of tumors. Among the elderly, the increase in tumor incidence and the decline in immune function show a clear synchronous trend.
It is well known that CD8+ T cells play a pivotal role in anti-tumor immunity. However, our understanding of how age-induced T cell dysfunction affects tumor control and the effectiveness of immunotherapy is still relatively limited.
In May 2024, Professor Debattama R. Sen's team at Harvard Medical School published a research paper titled "The aged tumor microenvironment limits T cell control of cancer" in the journal Nature Immunology. The study revealed that aging weakens the efficacy of CD8+ T cells in the anti-tumor process, thereby accelerating the development of tumors.

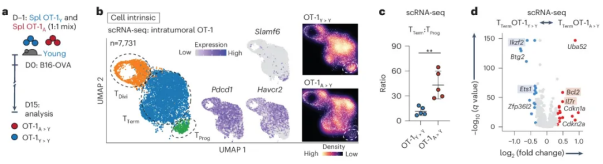
After in-depth research and analysis, researchers systematically explored the characteristics of mouse T cell aging. The results showed that during the aging process, the number of naive T cells showed a relatively decreasing trend, while PD-1+TIM-3+CD8+T cells showed an increasing trend. It is worth mentioning that these phenotypic changes showed a sharp increase trend when the mouse age was between 65 and 75 weeks (equivalent to 50 to 58 years old in human age). This discovery provides important clues for our in-depth understanding of the biological process of T cell aging.
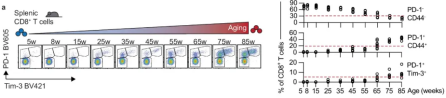
In subsequent experiments, the researchers implanted B16-OVA (melanoma cells) into C57BL/6J mice aged between 10 and 68 weeks. After careful observation and analysis, they found a close correlation between aging and a significant acceleration of tumor growth.
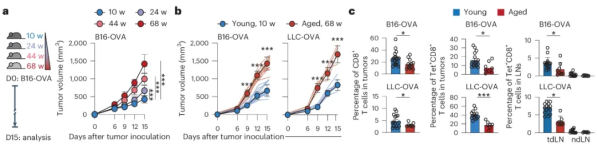
Subsequently, in order to further explore the impact of aging on tumor growth, the researchers further implanted B16-OVA or LLC-OVA cells (i.e., Lewis lung cancer cells expressing OVA) into young (10-12 weeks) and old (68-70 weeks) mice. After comparative experiments, they found that the tumor growth rate in old mice was faster than that in young mice, and the number of tumor-infiltrating CD8+ T cells in old mice was significantly reduced. In addition, the results also showed that the number of OVA-specific CD8+ T cells in tumors and tumor-draining lymph nodes of old mice also showed a downward trend.
Taken together, these data provide strong evidence for the negative impact of aging on tumor control and further reveal that aging broadly alters the fate and function of CD8+ T cells.
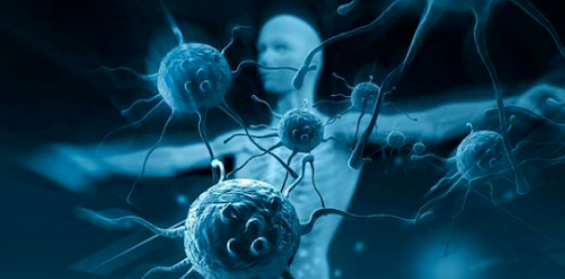
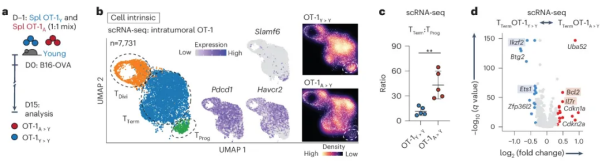
Next, the researchers wondered whether the age-related changes observed in tumor-infiltrating CD8+ T cells could be explained solely by baseline T cell intrinsic defects in healthy aged mice. To this end, the researchers co-transferred genetically marked CD45.1/CD45.2 young OT-1 (OT-1Y > Y) and CD45.1 old OT-1 (OT-1A > Y) CD8+ T cells into young wild-type (CD45.2) tumor-bearing mice and analyzed the tumor-infiltrating transferred T cells by scRNA-seq 15 days later.
The results revealed three major subsets, including known subsets of T Prog and T Term cells, which were defined by their unique gene signatures. In addition, the ratio of T Term cells to T Prog cells was much higher in aged OT-1 (A>Y) T cells than in OT-1 (Y>Y) T cells. Aged OT-1 (A>Y) T Term cells expressed less Ikzf2 and had higher expression of cell cycle inhibitors such as Cdkn1a and Cdkn2a. This suggests that CD8+ T cell dysfunction during aging is at least partially intrinsically imprinted within CD8+ T cells.
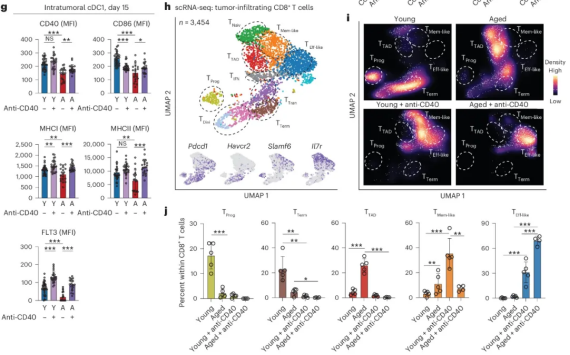
After in-depth research, we found that changes in the immune microenvironment of aging tumors impaired the interaction between natural killer cells (NK) and dendritic cells (DC) and CD8+ T cells, which in turn led to a reduction in the ability of traditional type 1 dendritic cells (cDC1s) to activate CD8+ T cells. Due to these defects, old tumor-bearing mice cannot obtain the expected therapeutic benefits from therapeutic mRNA vaccination. Ultimately, our research team found that bone marrow-targeted immunotherapy can activate cDC1s, thereby improving tumor control in elderly mice and significantly enhancing the immune response of CD8+ T cells.
In summary, this study revealed a close association between accelerated tumor growth during aging and decreased CD8+ T cell infiltration and function. Notably, due to the rapid induction of T cell dysfunction, transplanted T cells from young mice could not effectively restore tumor control in aged mice.
In addition, this study also found that in the aging tumor microenvironment, changes in NK-DC1-CD8+ T cell interactions impaired the T cell priming function of cDC1 and promoted the formation of T TAD cells. However, through bone marrow-targeted therapeutic approaches, traditional type 1 dendritic cells can be reactivated, thereby improving tumor control and restoring CD8+ T cell immune function in the aging process.