全球医讯

A new study published in Nature magazine revealed a new mechanism for abnormal accumulation of lipid droplets (LDAM) in microglia in the brains of patients with APOE4/4 genotype Alzheimer's disease. This mechanism shows that after being stimulated by amyloid protein (Aβ) and other innate immune factors, abnormal microglia promote phosphorylation of Tau protein and cell death in nerve cells. This discovery provides a new perspective for understanding how the APOE4 gene induces Alzheimer's disease and provides a basis for the development of potential therapeutic strategies.
APOE protein is an important lipid and cholesterol transporter, and APOE4 is an allele form that encodes this protein. This gene form changes the ability of APOE protein to transport cholesterol and lipids, and is closely related to the occurrence and development of Alzheimer's disease. In order to further study the relationship between APOE genotype and Alzheimer's disease, scientists performed single-cell nuclear RNA sequencing on the frontal cortex tissue of AD patients with APOE4/4 genotype. By comparing the transcriptome data of this patient group with those of AD patients with APOE3/3 genotype and healthy people with APOE3/3 genotype, the research team discovered the key role of abnormal accumulation of lipid droplets in microglia.
This discovery not only reveals a new mechanism by which the APOE4 gene induces Alzheimer's disease, but also provides new ideas for exploring effective treatments. By further studying this mechanism, scientists are expected to develop effective treatments for APOE4 genotype Alzheimer's disease, bringing new hope for the treatment of this serious neurodegenerative disease.
Image source: 123RF
After a detailed analysis of the microglial transcriptome, we found that the gene with the most significant change in expression level in the microglia of patients with APOE4/4 genotype AD was ACSL1. ACSL1 is a key lipid processing enzyme encoding gene that plays a vital role in the biosynthesis of lipid droplets. If ACSL1 is overexpressed, it will promote the formation of triglyceride-rich lipid droplets in various cell types. Further lipid staining analysis revealed that in the brain tissue of AD patients, there are more cells showing the accumulation of lipid bodies. It is worth noting that the number of lipid bodies in the cells is negatively correlated with the patient's performance in cognitive assessment, and the level of lipid bodies is positively correlated with the number of amyloid beta plaques and the pathological level of Tau protein.
In order to further explore the effect of APOE genotype on lipid droplet accumulation in microglia, the research team used advanced cell differentiation technology to successfully differentiate microglia (iMG) from induced pluripotent stem cells with APOE4/4 and APOE3/3 genotypes. Through lipid staining detection, we found that the degree of lipid droplet accumulation in microglia with APOE4/4 genotype was significantly higher than that with APOE3/3 genotype. More importantly, when these iMGs were stimulated by fibrillar Aβ, the accumulation of lipid droplets increased significantly, especially in cells carrying the APOE4 allele, where the accumulation of lipid droplets was more prominent. However, if the APOE gene is knocked out, fibrillar Aβ stimulation does not lead to the accumulation of lipid droplets. These findings provide us with a new perspective to deeply understand the pathogenesis of AD and provide important clues for the development of future treatment strategies.
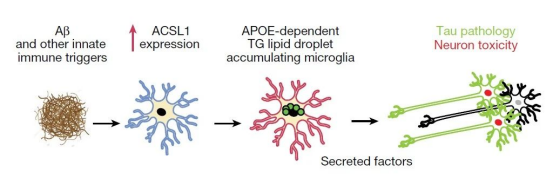
Further studies have shown that iMGs with lipid droplet accumulation can secrete neurotoxic substances, which lead to increased levels of phosphorylated Tau protein and cell apoptosis in surrounding neurons in cell culture experiments. Based on these data, the researchers proposed a new model of AD pathogenesis mediated by lipid droplet-aggregated microglia. In this model, Aβ activates triglyceride lipid synthesis, lipid droplet accumulation, and the release of neurotoxic factors in microglia. This series of processes depends on APOE. The released neurotoxic factors stimulate the hallmark features of neurodegenerative lesions.
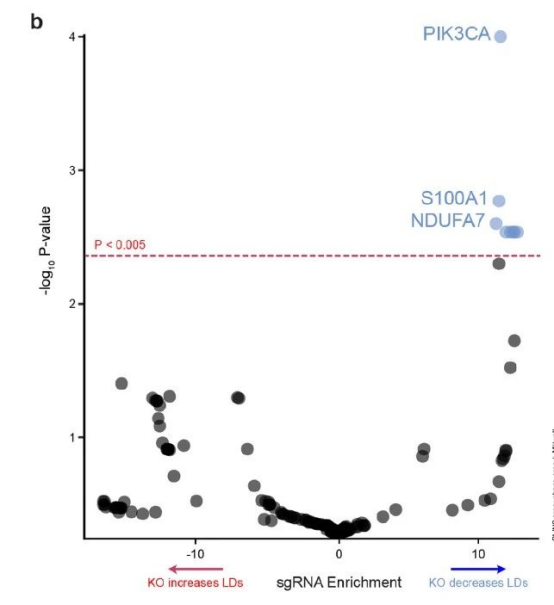
If lipid droplet accumulation in microglia is the key to triggering neurodegeneration, what means are there to reduce lipid droplet accumulation? To this end, researchers conducted CRISPR screening to find genes that can reduce lipid droplet accumulation. After screening about 2,000 druggable genes, the researchers found that the PIK3CA gene encoding the catalytic subunit of PI3 kinase (PI3K) is a genetic regulator of lipid droplet accumulation.
Inhibition of PI3K has been shown to regulate lipid droplet accumulation in macrophages in mouse models . The scientists decided to test whether inhibition of PI3K in human microglia had the same effect. The results showed that a small molecule PI3K inhibitor called GNE-317 significantly reduced lipid droplet production in APOE4/4 microglia after stimulation with fibrillar Aβ. Moreover, GNE-317 was able to reverse the secretion of proinflammatory cytokines in microglia that already had high levels of lipid droplet accumulation. Further studies showed that GNE-317 was able to reduce the expression of genes related to lipid synthesis and the production of proinflammatory cytokines, while increasing the expression of growth factors that degrade lipids, promote microglial homeostasis, and neuroprotection. The expression levels of genes related to autophagy were also increased after GNE-317 treatment. This means that GNE-317 may reduce lipid droplet levels by enhancing autophagy.
After in-depth exploration, researchers found that past studies on APOE focused on astrocytes, endothelial cells, neurons, and oligosynaptic cells, while the current study revealed a new mechanism by which APOE may induce Alzheimer's disease (AD) by regulating the accumulation of lipid droplets in microglia. In addition, inhibiting the PI3K signaling pathway in human microglia can effectively increase the expression level of autophagy-related genes and reduce the accumulation of lipid droplets. Although autophagy is regarded as one of the potential ways to delay the progression of neurodegenerative diseases, previous studies have mainly focused on autophagy-mediated protein degradation. Therefore, further exploration of the autophagy-mediated lipid degradation mechanism in microglia is expected to provide a new perspective for elucidating the pathogenesis of AD.
The researchers also pointed out that the impact of APOE genotype on AD pathology may involve a variety of different biological mechanisms, and different cell types may show their own unique modes of action in the development of AD. In the future, it is still necessary to further explore the interactions between various cell types and their contributions to the disease process in order to more fully understand the pathogenesis of AD.