全球医讯

Alzheimer's disease, commonly known as dementia, has become a global health challenge, and the economic and social burdens it brings are quite heavy. According to statistics, the number of dementia patients worldwide has reached nearly 50 million, and the number of new cases is about 10 million each year. In my country, the number of dementia patients is as high as tens of millions, ranking first in the world. Although the scientific community has invested a lot of research energy in this disease, no clear treatment plan has been found to effectively prevent or cure Alzheimer's disease. Therefore, in the face of this severe situation, we need to continue to increase scientific research efforts in the hope of finding more effective treatment strategies in the future to reduce the burden on patients and their families.
Alzheimer's disease (AD) is a complex disease whose pathogenesis covers a variety of rare and common genetic variations. Among them, mutations in three genes, APP, PSEN1 and PSEN2, are key factors leading to early-onset autosomal dominant Alzheimer's disease. In addition, mutations in dozens of other genes are closely associated with an increased risk of late-onset AD. It is worth noting that among these genetic risk factors, the APOE gene is generally considered to be the most significant factor. According to estimates worldwide, approximately 2-3% of the population carries two APOE4 genes, further increasing their risk of AD.
According to previous research results, those who carry two APOE4 genes, that is, APOE4 homozygotes, have a lifetime risk of developing Alzheimer's disease (AD) of 60% at the age of 85, which is significantly higher than the risk of heterozygotes or non-carriers.
On May 6, 2024, researchers from the São Paulo Institute in Spain published a research paper titled "APOE4 homozygozity represents a distinct genetic form of Alzheimer's disease" in the journal "Nature Medicine".
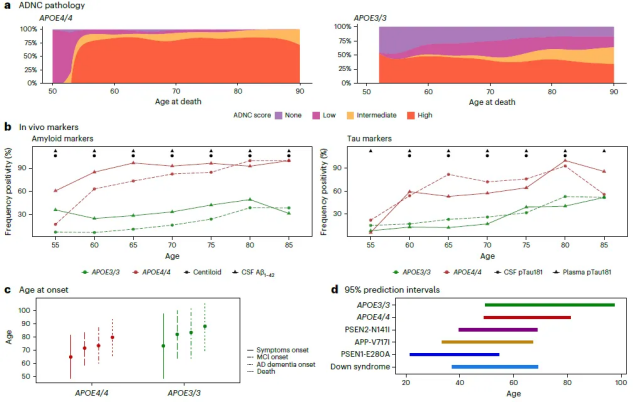
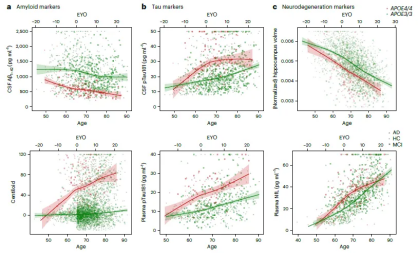
The study showed that almost all people carrying two APOE4s developed symptoms of Alzheimer's disease, and compared with APOE3 homozygotes, levels of AD biomarkers were significantly higher starting at age 55. By age 65, almost everyone had abnormal levels of amyloid in their cerebrospinal fluid.
This fully demonstrates that the APOE4 gene is not only a potential risk factor, but also the direct cause of the disease. If an individual has two copies of the APOE4 gene, it may represent a new genetic manifestation of Alzheimer's disease.

In this study, researchers conducted an in-depth analysis of the data from the National Alzheimer's Disease Coordinating Center and five large-scale Alzheimer's disease biomarker cohorts. These data cover a total of 3,297 pathological research individuals and 10,039 clinical research individuals, including 273 APOE4 homozygous samples and 519 APOE4 homozygous subjects with both Alzheimer's disease biomarkers. Through a systematic analysis of the clinical, pathological and biomarker changes of APOE4 homozygotes, the aim is to accurately assess their risk of developing Alzheimer's disease.
After in-depth research, we found that compared with APOE3 homozygotes, at the age of 55, almost all APOE4 homozygotes showed pathological characteristics of Alzheimer's disease, and the levels of their disease-related biomarkers were significantly increased.
According to rigorous scientific research, when individuals reach the age of 65, more than 95% of APOE4 homozygotes significantly show biological characteristics of Alzheimer's disease pathology in the brain, or the level of amyloid in the cerebrospinal fluid is abnormal. It is worth mentioning that up to 75% of individuals show positive results in amyloid scans. This discovery provides an important scientific basis for our in-depth understanding of the pathogenesis of Alzheimer's disease and the development of targeted prevention and treatment strategies.
Penetrance and predictive value of APOE4 homozygosity in AD
In addition, the study found that APOE4 homozygotes had an earlier age of symptom onset, at 65.1 years old.
Based on the above research results, the researchers solemnly pointed out that the genetic variation of the APOE4 gene is not only an important risk factor for Alzheimer's disease, but also very likely represents a special genetic pattern of Alzheimer's disease.
Changes in AD biomarkers with age in APOE3 and APOE4 homozygotes
The researchers further noted that the similarities between this redefinition of the disease and Down syndrome cannot be ignored. In particular, the predictability of symptom onset in APOE4 homozygotes, as well as the order of biomarker changes, show clear similarities to autosomal dominant AD and Down syndrome.
In addition, the researchers emphasized that existing data clearly show that if an individual has two copies of the APOE4 gene, this not only significantly increases the risk of developing Alzheimer's disease, but can also serve as an important marker for predicting the onset of the disease. Therefore, we urgently need to develop and implement specific prevention strategies to address this severe health challenge.
Taken together, the results suggest that having two copies of the APOE4 gene may indicate a new inheritance pattern for Alzheimer's disease. These findings have potentially important implications for developing personalized prevention programs, designing clinical trials, and developing targeted treatments for this specific patient population.
Paper link:
https://doi.org/10.1038/s41591-024-02931-w