全球医讯
Alzheimer's disease is the most common neurodegenerative disease among the elderly. Of particular concern is that those who carry the APOE-ε4 allele have a significantly increased risk of developing the disease. According to scientific research, if an individual carries one APOE-ε4 allele, their risk of disease will increase by 2-3 times; if they carry two APOE-ε4 alleles, the risk of disease will increase to 8-12 times. However, it is worth noting that although some people carry two APOE-ε4 alleles, they are still able to survive the invasion of Alzheimer's disease, as if their bodies are naturally "resistant" to the disease.
Recently, a research team led by scientists from Columbia University revealed the reasons for protecting this special group of elderly people from Alzheimer's disease in an in-depth study. This discovery not only provides us with a new perspective to understand this disease, but also opens up new possible avenues for research on the prevention and treatment of Alzheimer's disease. The researchers believe that this important discovery may become a new target for future research on the prevention and treatment of Alzheimer's disease.

This study conducted DNA sequence analysis on healthy elderly people over 70 years old who carried two APOE-ε4 alleles and found that their DNA was commonly associated with gene variants related to the extracellular matrix (ECM) signaling pathway, especially the rare variant rs140926439 on the FN1 gene. This variant can reduce the risk of Alzheimer's disease and delay the onset of the disease. The study also found that the number of APOE-ε4 alleles was positively correlated with the level of fibronectin in the BBB, but the level of fibronectin in the BBB of the elderly carrying the FN1 gene variant was not significantly increased. The researchers speculated that the level of fibronectin in the BBB may be related to the deposition of amyloid in the brain, which is one of the typical characteristics of Alzheimer's disease.

Image source: 123RF
After careful experimental verification, the research team knocked out the homologous gene corresponding to the human FN1 gene in the zebrafish Alzheimer's disease model . The experimental results show that after the loss of this gene, the ability of zebrafish to clear amyloid proteins has been significantly improved. At the same time, the activity of microglia responsible for clearing amyloid proteins has also been enhanced. In addition, pathological changes such as gliosis similar to those in Alzheimer's patients in zebrafish have also been alleviated. Further analysis of the rs140926439 variant showed that this variant may lead to the inactivation or functional impairment of fibronectin. Based on the above experimental results, the research team believes that the rs140926439 gene mutation on the FN1 gene may enhance the brain's ability to clear amyloid proteins by inactivating fibronectin, reducing its deposition level in the BBB (blood-brain barrier), and activating the activity of microglia, thereby achieving the potential effect of preventing the occurrence of Alzheimer's disease.
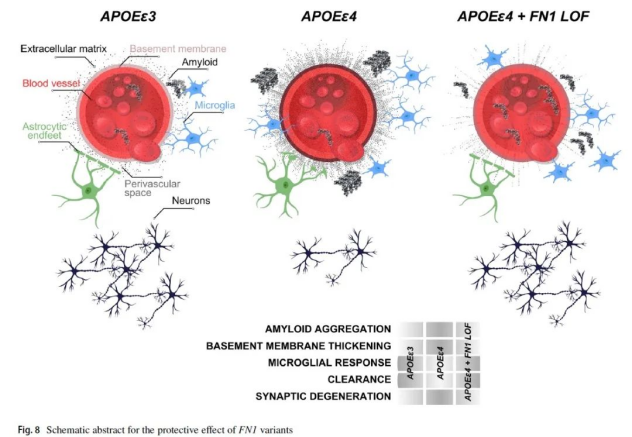
The researchers pointed out that although this specific gene mutation was discovered in an analysis of people carrying the APOE-ε4 allele, its potential impact may extend to a wider population. Existing studies have revealed that there are significant differences in the levels of BBB fibronectin deposition between people with normal cognitive abilities and patients with Alzheimer's disease, regardless of whether they carry the APOE-ε4 allele. Therefore, treatments aimed at reducing fibronectin deposition may also have a positive effect on people carrying other types of APOE alleles.
In the field of human genetics research, the strategy of developing new drugs for specific targets has achieved remarkable success. For example, PCSK9, an important target for the treatment of cardiovascular disease, was first discovered in 2003. At that time, researchers observed that mutations in the PCSK9 gene would lead to increased expression levels of PCSK9 protein, which in turn promoted the increase of low-density lipoprotein cholesterol (LDL-C), and may eventually cause familial hypercholesterolemia (FH). Soon after, another research team reported that there were naturally gene mutations that inhibited PCSK9 expression in a small number of people. These people had extremely low LDL-C levels, significantly reduced incidence of cardiovascular disease, and no other adverse effects were shown.
Antibody therapy and RNAi therapy targeting PCSK9 have been approved by the US FDA for marketing. Oral small molecule therapy and gene editing therapy that can permanently reduce PCSK9 protein expression are also in the clinical development stage and have shown positive therapeutic prospects. We hope that the new targets for Alzheimer's disease revealed in the current study can be further verified in subsequent studies, thereby promoting the development of innovative therapies and bringing good news to more patients.